Invention for Genetically Modified Human Natural Killer Cell Lines
Invented by Kerry S. Campbell, Institute for Cancer Research
Genetically modified human natural killer cell lines are created by introducing specific genetic modifications into the cells to enhance their anti-tumor activity. These modifications can include the introduction of chimeric antigen receptors (CARs) or the overexpression of cytokines and chemokines that enhance the cells’ ability to recognize and kill cancer cells.
The market for genetically modified human natural killer cell lines is driven by the increasing prevalence of cancer and the need for more effective treatments. According to the World Health Organization, cancer is the second leading cause of death globally, with an estimated 9.6 million deaths in 2018. Traditional cancer treatments such as chemotherapy and radiation therapy can have significant side effects and are often ineffective in advanced stages of the disease. Genetically modified human natural killer cell lines offer a promising alternative for cancer treatment with fewer side effects and potentially higher efficacy.
The market for genetically modified human natural killer cell lines is also driven by the increasing investment in research and development in the field of immunotherapy. In recent years, there has been a significant increase in the number of clinical trials investigating the use of genetically modified human natural killer cell lines for the treatment of various cancers. These trials have shown promising results, with some patients achieving complete remission of their cancer.
The market for genetically modified human natural killer cell lines is highly competitive, with several companies and academic institutions actively developing and commercializing these products. Some of the key players in the market include Fate Therapeutics, NantKwest, and Celularity. These companies are investing heavily in research and development to improve the efficacy and safety of their products and to expand their applications to other diseases beyond cancer.
In conclusion, the market for genetically modified human natural killer cell lines is a rapidly growing field with significant potential for the treatment of cancer and other diseases. The increasing investment in research and development, coupled with the growing prevalence of cancer, is driving the demand for these products. As the field continues to evolve, it is likely that we will see more innovative products and therapies emerge, offering new hope for patients with cancer and other diseases.

The Institute for Cancer Research invention works as follows
The invention is a modified natural killer cell (NK-92) that expresses an Fc receptor at the cell surface, such as CD16(Fc?RIII?A), or another Fc? Fc receptors or Fc receptors. The modified NK92 cell can also be modified to express an accessory signaling proteins, such as TCR-? or Fc?RI?-? or to express interleukin-2 or other cytokines. “Additional methods are disclosed for a variety of assays and assessments, therapeutic treatments, or cytokines with the modified NK92 cells.

Background for Genetically Modified Human Natural Killer Cell Lines

NK cells are a type of lymphocyte that makes up approximately 10% of all lymphocytes found in humans. NK cells are primarily responsible for providing an innate cellular response against infected cells and tumors. Other roles in priming, regulation of humoral immunity response, fetal growth and elimination of stressed or injured normal cells were demonstrated and/or considered likely. The NK cells that are CD3?/CD56+ have a wide range of activating and inhibiting cell surface receptors. When an activating NK-cell receptor binds to the corresponding ligand of a target, the NK-cell is triggered to produce a cytotoxic response against that target and secrete a number of cytokines which perform functions like stimulating and recruiting other immune system elements to attack the target. Activated NK cell lyse targets via secretion and activation of the enzymes granzyme and perforin, as well as other mechanisms that are less well characterized.
NK cell inhibiting receptors engage primarily with major histocompatibility class I (?MHC-1?) The proteins that cover the surface of normal cells are called MHC-I. These inhibitory receptors, when engaged, prevent NK-cell activation. The MHC molecules are responsible for defining cells as “belonging” to a particular individual. to a particular individual. It is believed that NK cells are only activated by cells which express these MHC I molecules. MHC-I molecules can be missing or deficient, as they are in many tumors or viruses. The phenotype of NK cells and their activation pattern is distinct from the pattern exhibited by cytotoxic lymphocytes (?CTLs?). The CD3+/CD56+/CD8+ phenotype is activated by cells that have small foreign peptides from tumor cells or viruses attached to MHC-I molecules. Scientists speculate that NK cells developed as a response against tumors and infected cell that evade CTL destruction through suppression or disruption to the display of peptide presenting MHC I molecules.
NK cells are being evaluated as a potential therapeutic agent for the treatment of cancer. The NK cells are isolated from peripheral blood lymphocytes (?PBL?) The NK cells used for this purpose are isolated from the peripheral blood lymphocyte (?PBL?) The results of this treatment have been promising but the preparation of autologous NK-cells is costly, laborious and time consuming. Furthermore, the quality control of these NK-cells is complicated because each preparation is subject specific. The quantity of NK-cells that can be extracted from a given subject can vary significantly, and the cells can often lack proliferative and/or cytotoxic ability. The presence of antigens that are on the surface of the cells can trigger an immune rejection reaction when they are infused in a different subject than the one where the cells were isolated. It is therefore necessary to perform a careful MHC-I match between the donor’s and recipient’s MHC, as well as immunosuppressing the recipient.
The NK-like cell NK92 was found in the blood of a patient with non-Hodgkins Lymphoma. NK-92 cell lines lack the inhibitory receptors of normal NK cells but retain most activating receptors. The characterization of the NK92 cell line by Gong et. al. (1994; Yan et. al., 1998) showed that NK92 cells were cytotoxic towards a much broader range of tumors and infected cells than NK cells. They also displayed higher levels of cytotoxicity. NK-92 cell do not attack normal cells or elicit a rejection immune response. NK-92 can also be easily and stably maintained in continuous culture, allowing for large-scale production of NK-92 under cGMP-compliant quality control. NK-92 has been entered in clinical trials that are currently ongoing for multiple types of cancers because of this combination of characteristics.
While NK-92 cell retains almost all activating receptors, cytolytic pathways, and cytotoxic pathways associated with NK, they lack the CD16 receptor, and therefore cannot lyse targets cells via the ADCC mechanisms. NK-92 cell cannot, despite all their benefits, potentiate endogenous and exogenous antibody effects against tumors or infections in the same way as NK cells. NK92 is not the only NK-like line. Some of the other NK cell lines also express CD16 but it is instabile. The cells are difficult to grow and exhibit weak cytotoxic activity. “For these reasons, only NK92 is a viable therapeutic candidate, despite the fact that it does not contain CD16.
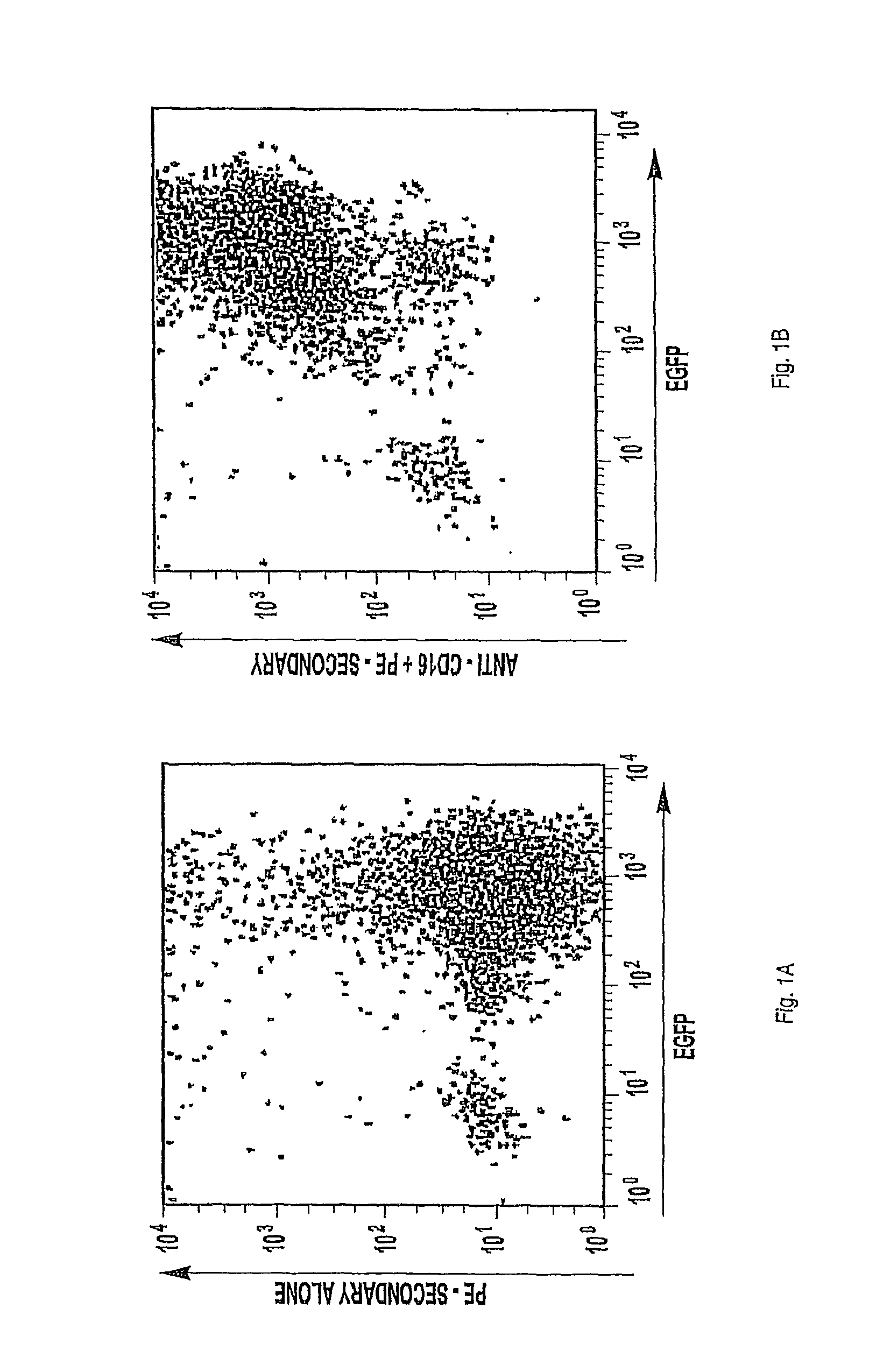
It would therefore be advantageous to restore CD16 to NK92 cells and to allow them to act through the ADCC mechanisms, allowing these cells to be used with antibodies in conjunction for therapeutic purposes and other related uses. NK cell line recalcitrance to gene transfer has hindered the development of these cell lines for therapeutic or research purposes. Using particle-mediated gene transduction or retroviral transfer, only 5-15% of NK-92 cell transformation efficiencies were achieved (Nagashima et. al. 1998; Tam et. al. 1999). NK-92 cells that express the CD16 receptor on their cell surface are not available at this time.
The various embodiments of this invention use or provide an NK92 cell that has been modified to express a Fc-receptor such as CD16(Fc?RIIIA), or, more generally, any Fc-receptor, on the cell’s surface.
The invention relates to NK-92 modified cells that express a Fc on the surface of the cell. The Fe receptor can either be an activating Fc or any member of an Fe receptor class, such as FC?RI (CD64), FC?RII (CD32), FcRIII, FcRn, FcRnA and FcRnA. “In a first aspect, the invention is directed to NK-92 cells modified to express a Fc receptor on a surface of the cell; The Fe receptor can be an activating Fc?RIII?A receptor, CD16, or any member from fc receptor classes, such as FCRn (CD64), FCRII (CD32), FCRIII, FC?RIII (FcRn), FcRIII, and Fc? FcRn, Fc? The Fc receptors may have any affinity to bind their ligands or fragments thereof, including low and high affinity forms. NK-92 can be modified through the introduction of a polynucleotide encoding a polypeptide that has at least 70%, 80% or 90% identity to SEQID NO:1 or 2; one polynucleotide is SEQID NO:3. The NK92 cells can also be modified to express associated accessory signaling cytokines or fragments of cytokines. This expression can correlate with an increased surface expression for the Fc receptor. The associated accessory signaling polypeptides are Fc?RI? Fc?RI-? TCR-? (SEQID NO:7). The expression of a cytokine, such as interleukin-2, can also correlate to the viability or cytotoxicity (of modified NK92 cells).
The invention can be described as a method for in vitro assessment of an antibody’s efficacy to induce cell death. These methods may include exposing a cell target to an antigen (monoclonal or polyclonal), exposing a cell target to a modified NK92 cell expressing a Fc receptor, and monitoring the cell target for cytotoxicity or cytolysis or apoptosis. Multiple cells and antibodies may be used. In the absence of antibodies, the target cells can be lysed or aptotic at a rate between 5%-30% when the modified NK92 cells are present. The ratios of effector to target can range from 0.5:1 up to 100:1, with 1:1 and 20:1. The methods can modify SKOV-3 cells, THP-1 cells, T98G cells, A ML193, SR91 cells, REH cells, and other cells to increase the expression of the antigen that the antibody binds. Appropriate negative controls include using unmodified NK-92 cells.
The NK-92 cell in this aspect may include those that have been modified to express a Fc on the surface of the cells; the Fc can be an activating Fc. “The NK-92 cells in this aspect can include those modified to express a Fc receptor on a surface of the cell; the Fc receptor can be an activating Fc?RIII?A receptor, CD16, or any member from a Fc receptor family, such as FCRn (CD64), FCRII (CD32), FCRIII, FcRn (FcRIII), FcRn (FcRIII), FcRIII, FcRn (Fc? FcRn, Fc? The Fc receptors may have any affinity to bind their ligands or fragments thereof, including low and high affinity forms. The NK92 cells can also be modified through the introduction of a polynucleotide encoding a polypeptide that has at least 70%, 80% 90%, 95% 99%, or 100% identity with the amino acid sequences in SEQID NO:1 or NO:2. One such polynucleotide is SEQID NO:3. The NK92 cells can also be modified to express associated accessory signaling cytokines or fragments of cytokines. This expression can correlate with an increased surface expression for the Fc receptor. The associated accessory signaling polypeptides are Fc?RI? Fc?RI-? TCR-? (SEQID NO:7). The expression of a cytokine, such as interleukin-2, can also be correlated with the viability or cytotoxicity (of modified NK92 cells). The assay can be supplemented with cytokines from external sources.

The invention is directed, in a third aspect to methods of detecting cytolytic activity and apoptosis, which include the steps of exposing a cell target to a NK92 cell expressing a Fc receptor and monitoring the cell target for cytotoxicity or cytolysis. Monitoring can include determining IFN-? Monitoring can include determining IFN-? “The method can also include blocking agents such as receptor-masking polypeptides or antibodies (or fragments thereof) that activate receptors to suppress one of more activating NK-92 cells.
The invention, in a fourth aspect is directed at methods for assaying efficacy of antibodies to treat a lesion or tumor. This method includes the steps of administering a plurality of antibody to a patient; administering modified NK92 cells expressing a Fc receptor to a patient; and monitoring the lesion or tumor. The treatment’s efficacy correlates to the suppression of tumor, infection or lesions in the subject. Monitoring can include determining IFN-? Monitoring can include determining IFN-? “The method can also include blocking agents such as receptor-masking polypeptides or antibodies (or fragments thereof) that activate receptor-masking receptors to suppress one of more activating cells on the NK92 cell.
The antibody can be monoclonal or polyclonal. It can also be chimeric, such as a chimera with at least two antigen-binding domains that are dissimilar (one of which can be adapted so it binds the Fc receptor), and any other type. The cytokines (such interleukin-2) or fragments of them can be expressed by modified NK92 cells, or provided exogenously. The subjects include canines, felines, ferrets and mice. The subjects can also be humans.
The NK-92 cell in this aspect may include those that have been modified to express a Fc on the surface of the cells; the Fc can be an activating Fc. “The NK-92 cells in this aspect can include those modified to express a Fc receptor on a surface of the cell; the Fc receptor can be an activating Fc?RIII?A receptor, CD16, or any member from a Fc receptor family, such as FCRn (CD64), FCRII (CD32), FCRIII, FcRn (FcRIII), FcRn (FcRIII), FcRn (FcRIII), Fc FcRn, Fc? The Fc receptors may have any affinity to bind their ligands or fragments thereof, including low and high affinity forms. The NK92 cells can also be modified through the introduction of a polynucleotide encoding a polypeptide that has at least 70%, 80% 90%, 95% 99%, or 100% identity with the amino acid sequences in SEQID NO:1 or NO:2. One such polynucleotide is SEQID NO:3. The NK92 cells can also be modified to express associated accessory signaling cytokines or fragments of cytokines. This expression can correlate with an increased surface expression for the Fc receptor. The associated accessory signaling proteins include Fc-RI-? The accessory signaling polypeptides include Fc?RI-?
In a fifth and final aspect, the invention is directed at methods for treating a patient, the patient having a lesion or tumor. The method includes administering to a patient antibodies that specifically bind the lesion or tumor. A decrease in the lesion, tumor or infection indicates a positive therapeutic response. Monitoring can include determining IFN-? Monitoring can include determining IFN-? “The method can also include blocking agents such as receptor-masking polypeptides or antibodies (or fragments thereof) that activate receptors to suppress one of more activating proteins on the NK92 cell.
The antibody can be monoclonal or polyclonal. It can also be chimeric, such as a chimera with at least two antigen-binding domains that are dissimilar (one of which can be adapted so it binds the Fc receptor), and any other type. The cytokines (such interleukin-2) or fragments of them can be expressed by modified NK92 cells, or provided exogenously. The subjects include canines, felines, ferrets and mice. The subjects can also be humans.
The NK-92 cell in this aspect may include those that have been modified to express a Fc on the surface of the cells; the Fc can be an activating Fc. “The NK-92 cells in this aspect can include those modified to express a Fc receptor on a surface of the cell; the Fc receptor can be an activating Fc?RIII?A receptor, CD16, or any member from a Fe receptor class such as FCRII, FC?RIII (CD32), FCRn, FC?RIII (CD64), FCRn, FC?RIII (CD32), FCRIII, FCRn, FC? Fc8. The Fc receptors may have any affinity to bind their ligands or fragments thereof, including low and high affinity forms. The NK92 cells can also be modified through the introduction of a polynucleotide encoding a polypeptide that has at least 70%, 80% 90%, 95% 99%, or 100% identity with the amino acid sequences in SEQID NO:1 or NO:2. One such polynucleotide is SEQID NO:3. The NK92 cells can also be modified to express associated accessory signaling cytokines or fragments of cytokines. This expression can correlate with an increased surface expression for the Fc receptor. The associated accessory signaling proteins include Fc-RI-? The accessory signaling polypeptides include Fc?RI-?
The following detailed description and embodiments of the invention, the claims and the accompanying drawings will make it clear that the present invention has many other features and advantages.
The present invention can be embodied in many different ways. However, specific embodiments are illustrated in the drawings and described in detail herein. It is understood that this disclosure is intended as an illustration of the principles and not to limit the invention only to the specific examples shown.
The present invention relates to cell lines and methods which enhance and expand the effectiveness of the ADCC reaction. The present invention is an NK92 cell line stably expressing an Fc surface receptor protein such as CD16. “(Several different names have been used to describe certain Fc receptors. They are all interchangeable here, including CD16 and FC?RIIIA, as well as their polymorphisms, or other forms with varied affinity levels.)

Click here to view the patent on Google Patents.


